Some of us may remember a day when ketchup was placed in thick glass bottles with skinny necks. I’d like to meet the person who came up with that idea and kindly ask, “What were you thinking?” How many of us would sit in diners hitting the bottom of the bottle with the heel of our hand or shake it aggressively, finally giving up and jabbing a butter knife into the neck and making a big ketchupy mess? Whoever decided to put the ketchup in the bottle and play that joke on us may or may not have realized that ketchup is a special type of fluid known as thixotropic, meaning it gets thinner as it is shaken.
Another thixotropic fluid, although less common table fare than ketchup, is pudding. Think back to your own formative years, sitting in a high chair with a bib. Your caregivers may have put a bowl of pudding-like food in front of you. As you play with your food, trying to coordinate the use of a spoon something may have put in your hand, you may have mixed it around or slung a bit here and there. The more you mix, the thinner it gets. As you sling it around you notice that it thickens up again, maybe even caking up on the wall or your parent’s clothes.
A thixotropic fluid is one that exhibits characteristics of thixotropy. Thixotropy is defined as a time-dependent shear thinning property. Put simply, after a thixotropic fluid starts to move it gets thinner, its viscosity decreases and it starts to flow more freely. One of the most fascinating things about a thixotropic fluid is that it can return back to its more viscous state. Thixotropy arises because the particles in the fluid require time to re-organize. Just like materials change their physical state based on temperature, going from liquid to solid to gas; fluids like ketchup and pudding change their physical viscosity (thickness or thinness) based on how much they are agitated.
Viscosity is the measure of a fluid’s resistance to flowing at a given rate. It is measured in mass per unit area at a certain rate, known as the rate of shear deformation. There is a whole plethora of units used to describe viscosity, or how thick a fluid is. The list includes units like Poise, Stokes, Saybolt Seconds Universal and Degree Engler. The formula for viscosity is:
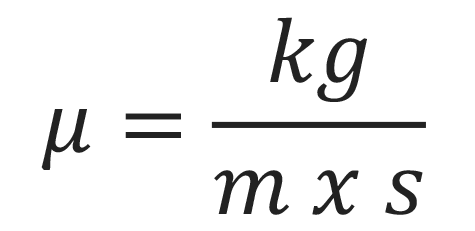
The Poise has a relatively logical definition as it is the “viscosity of a fluid in which the tangential force of 1 dyne per square centimeter maintains a velocity of 1 centimeter per second between two parallel planes that are 1 centimeter apart.” I imagine the movement as if you were to slowly push a dice across the table with your index finger. If you take that force you used to push the dice and divide it by 100, you get 1 centipoise and this is the viscosity of water. Another facet of viscosity is the density of a fluid. If you take viscosity and divide it by the density you get units in Stokes and centistokes. Once again, the kinematic viscosity of water is about 1 centistoke, but comparatively the kinematic viscosity of oil would be less than the dynamic viscosity since the density is less.
Stepping outside the realm of metric units we have a couple of units that involve test procedures developed by ASTM (American Society for Testing and Materials). ASTM D88 is the empirical method for determining the Saybolt Universal viscosity, specifically for petroleum products. For reference, the SSU (Saybolt Second Universal) viscosity of water is about 31 SSU. In classic imperial fashion, the test method is unique unto itself. The test method utilizes a viscometer developed by George Saybolt and utilized by Standard Oil starting in the 1880s. Basically, 60 milliliters of fluid is placed in the device and allowed to flow through a calibrated tube. Voila, the time it takes for the fluid to pass through the tube in seconds is your viscosity in SSU. Since 60 ml is only a couple of ounces and the viscosity of water is 31 SSU, that calibrated tube must be pretty small in diameter. Degree Engler goes back further than Saybolt Seconds and is basically a ratio of the time it takes for 200 ml of a fluid to flow through a tube relative to water.
One of the most common references to viscosity is when we go to change our motor oil. Motor oil is not thixotropic but its viscosity does change with temperature. Engine oil is truly an engineered fluid since you want it to be thin when you start your engine but not get too thin as it heats up. The Society of Automotive Engineers (SAE) has developed a viscosity rating system where the number proceeding the “W” is for winter conditions, or cold starting and the second number is the viscosity at operating conditions. For reference, a 10W-40 motor oil will have a Saybolt viscosity of around 125 SSU at 100° F and not get much thinner at higher operating temperatures. I’m no expert by any means on this matter. If you wanted an expert explanation, you would have to ask a “tribologist,” an expert in the science and engineering of interacting surfaces in relative motion.
Besides the fact that is interesting to learn about fluid properties, how are thixotropy and viscosity relevant to plumbing and mechanical engineering? Probably the best example is when we apply glycol mixtures for freeze protection. Glycol mixtures have a higher viscosity which affects pipe, pump and coil sizing. The other example is fuel oil. Knowing the type of fuel oil may determine if additional clearances are required in the pumping equipment.
One of the most fascinating things about engineering is how we define the properties of materials with math, formulas and units. If you are like me, mathematics can be intimidating. I like to see and feel something to know how it acts. For when you have direct experience with all of the things that we design you can truly understand that the proof is in the pudding.



