Most of us have likely heard the expression “ignorance is bliss.”
It is meant to imply that if you don’t know about something, you won’t spend time worrying about it. This expression came to me as I was thinking about the wonder of vacuum. Being completely ignorant is kind of like having an empty mind, and emptiness is really what happens in a vacuum: a space devoid entirely of matter.
Plumbing engineers design systems with vacuum for medical, laboratory and practical applications. When you stop to think about what vacuum systems are used for — surgery in hospitals, suction in dental offices and environmental control for laboratory experiments — you realize how important vacuum systems are.
Most applications I’ve worked on utilize a vacuum at around 19 inches of mercury. This is equivalent to around 9.5 psi below atmospheric pressure. Other units of vacuum include the Torr, millibar and pascal. While we commonly use inches of mercury here in the United States, the Torr deserves credit as the original unit of vacuum measure. One Torr is the same as 1 millimeter of mercury named after Evangelista Torricelli, an Italian physicist and mathematician who discovered the principle of the barometer in 1644.
If you imagine an actual manometer, a device used to measure vacuum, you can understand why vacuum has historically been measured using millimeters or inches of mercury. Utilizing mercury, the device is just small enough to sit on a laboratory bench or hang on the wall. If Torricelli were to try to measure vacuum using inches of water, a 100-millimeter change in vacuum of mercury (100 Torr) would require a manometer that was 54 inches high. 100 Torr corresponds to around 4 inches of mercury. Below are some conversions for a typical medical vacuum system, set at 19 inches Hg, to help visualize vacuum units mathematically:

One of the fascinating things about designing a vacuum system is the fact that air is compressible. Let’s say we design the piping distribution to lab and medical vacuum outlets throughout a facility. A 2-inch pipe can handle 25 standard cubic feet per minute (SCFM) with a 0.3-inch Hg drop in pressure per 100 feet. According to Figure 10-4 in the ASPE Design Handbook, Volume 2, this equates to about 100 lab outlets. The important thing to know is that this 25 SCFM can expand dramatically on the exhaust side of the vacuum pump. For a typical vacuum system operating at 19 inches Hg, the air flow will expand 3 times to about 75 actual cubic feet per minute (ACFM). A specialized lab application that could require 26 inches Hg would cause the SCFM to expand close to 8 times from 25 SCFM to 192 ACFM. Referring to Table 10-11 shows that we would need a 4-inch diameter exhaust pipe to accommodate 25 SCFM under 26-inch Hg vacuum through a 2-inch vacuum main. The formula for converting SCFM to ACFM is outlined in the ASPE Design Handbook, Volume 2, Equation 10-1 as:
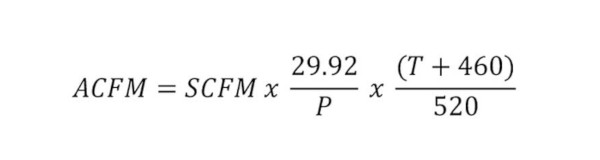
Perfect vacuum?
One of the most interesting things about vacuum is that there is a level of “perfect vacuum” that only exists theoretically in outer space. This is one of the reasons we need a spacesuit when we travel outside of a spacecraft. Besides the fact that there is no life-sustaining air to breathe, the atmospheric pressure that we enjoy on Earth is what keeps the fluids in our body from “boiling,” not due to heat, but “boiling” from reduced pressure.
Similarly, temperature can reach a low point where all motion stops. Imagine a state where atomic motion stops because it is so cold? This happens at 0° Kelvin or -460° F. It’s interesting that vacuum and temperature reach points where they can go no lower, but when they go in the other direction they seem to increase to infinite levels. The sun itself clocks in at 27-million degrees and the highest known theoretical temperature is around 142 nonillion. For those of you, like myself, who have never heard of a nonillion, it is bigger than an octillion but smaller than a quattuordecillion (that should clear things up). This theoretical highest-known temperature is thought to have occurred at the special event known as the “Big Bang.”
Let’s fast forward through the 17th, 18th and 19th centuries and look at the units used in the Metric system, the Pascal. In the early 20th century as weather forecasting became more of a science, Vilhelm Bjerknes developed the use of the millibar to help predict weather. Conveniently neat, as metric units usually are, the scale from perfect vacuum to atmospheric pressure goes from 0 to 100 kilo Pascal, which is also 0 to 1,000 millibar. Pressure, which is really what we call vacuum when it is positive, is what keeps us comfortable in our travels. This pressure, under standard conditions at sea level, is 14.7 psi. This is also known as 1 atmosphere, or around 1,000 millibars. Even slight changes in pressure can cause our ears to pop or let us know what the weather will bring. A change in 1.0-inch Hg or just 30, is enough to get a hurricane spinning.
Ultimately, pressure and vacuum are the same thing; one is just the absence of the other. Just like hot and cold are opposites. Life itself is a lot like the relationship between pressure and vacuum; hot and cold. All of us can get “hot-headed” at times or “keep our cool.” The pressures of everyday life can become overwhelming and fill our minds with racing thoughts. I’d like to propose a new way to visualize the scale of vacuum — the amount of thoughts that clutter our minds. I think a 0 to 1,000 scale works well where 0 is a completely empty mind, possibly achieved through years of transcendental meditation. We can’t always obtain bliss through ignorance because we’ve filled our heads with knowledge and experience. Other times, we can clear our minds by doing things we enjoy, such as a nice walk on the shore or enjoying a favorite meal with somebody close.
A little bit of pressure is what keeps life interesting, but every now and then it’s nice to clear the mind; as long as we don’t live in a total vacuum.



