Microbial quality of building water systems has increasingly become a focus of attention by regulatory authorities and the scientific community.
An example of this is the Centers for Medicare and Medicaid Services memorandum “Requirement to Reduce Legionella Risk in Healthcare Facility Water Systems to Prevent Cases and Outbreaks of Legionnaires’ Disease,” which applies to Medicare-certified hospitals, critical access hospitals, and long-term care. It establishes a requirement for each facility to conduct a risk assessment to identify where Legionella and other opportunistic pathogens could grow and spread in the building water system.
The memorandum also requires that facilities implement a water management program that considers ASHRAE Standard 188, including control measures and environmental testing for pathogens. There are also regulations, such as the Safe Drinking Water Act, which include waterborne organisms (heterotopic plate count, total coliforms, Legionella, cryptosporidium, giardia lamblia and enteric viruses) that public water systems need to consider.
An association with water may be one of the few similarities between these waterborne organisms as their source, growth factors, susceptibility to disinfection, survival outside of water, mode of transmission and type of infection all differ. These differences are important considerations when designing building water systems and assessing disease risk in a facility.
It is also important to understand that because of these differences, the control measures established in water safety and management programs (WSMPs) for Legionella may not be effective for control of these other organisms. Simply lumping Legionella and other waterborne bacteria together in a WSMP, with the assumption if you control one, you control all, is not a prudent approach for building water systems.
Engineers and building professionals need to understand a little about the microbiology and epidemiology of these organisms to understand the role building water systems may play in disease risk, and to properly manage that risk.
Heterotrophic plate count
Heterotrophic plate count (HPC) is a term used to describe a range of bacteria commonly found in drinking water. These bacteria all utilize organic carbon for growth, can be detected by culture and are naturally occurring in all water systems. Sometimes referred to as total heterotrophic aerobic bacteria (THAB) or by other names, these bacteria are common in potable building water systems with literature reporting up to 300,000 colony-forming units per milliliter in potable water. The World Health Organization, Environmental Protection Agency and peer-reviewed literature indicate that HPC cannot be used as an indicator for waterborne pathogens (does not detect Legionella), and that there is no correlation between HPC and the risk for disease. HPC can be used to monitor for changes in water quality or treatment efficacy, and is sometimes specified as a performance standard for evaluating disinfection of building water systems during the commissioning process. However, the results of an HPC test should not be used as an indicator of a pathogen-free water system or as a validation point in a WSMP.
The other waterborne pathogens can be divided into two broad groups: fecal and opportunistic pathogens. Waterborne fecal pathogens include coliforms, such as E. coli, cryptosporidium, giardia, enteric viruses and others. These organisms typically require an animal host to reproduce, and while they can persist in a water system, they do not grow.Water treatment systems are designed and monitored to remove or inactivate these fecal pathogens through filtration and disinfection.
By providing a residual disinfectant in the distribution system, public water systems minimize the potential for recontamination of the water supply. Commissioning requirements often include a process of chlorinating new water systems to manage risk associated with fecal pathogens. A test, such as total coliform analysis, often is performed to demonstrate that a water system is free of fecal pathogens prior to use or occupancy.
Other opportunistic pathogens
There are numerous waterborne other opportunistic pathogens (OOP), but for the purpose of this article these will be limited to the bacteria included in the CMS policy (pseudomonas, acinetobacter, burkholderia, stenotrophomonas, and nontuberculous mycobacteria). Unlike the fecal pathogens, OOP may not be completely removed or inactivated in the public water treatment process, do not require an animal host to reproduce, and can amplify in the distribution or building water system. Optimal growth conditions often are found in building water systems where there are abundant nutrients, low or no disinfectant residual, tepid or warm water temperatures, and the presence of other microorganisms. There is no indicator test, such as HPC or coliforms, that can be used to determine the presence or absence of OOP in a water system. For example, to validate a WSMP for Legionella requires environmental testing for Legionella to confirm its growth is under control.
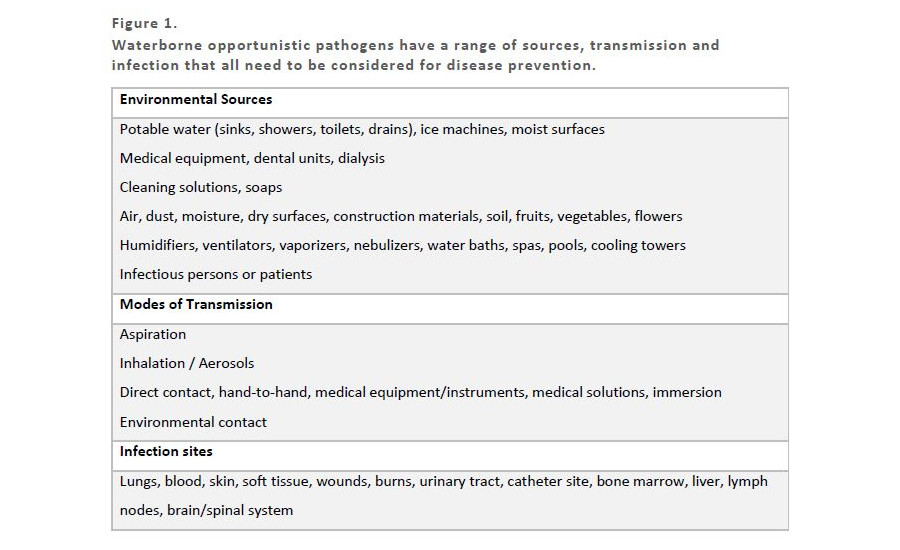
Preventing disease from OOPs requires management of the environmental source, mode of transmission and infection site. ASHRAE Standard 188 “Legionellosis: Risk Management for Building Water Systems” prescribes that managing Legionellosis can be effectively performed by implementing a WSMP. This is because Legionella’s source (building water), mode of transmission (aspiration or aerosolization) and infection site (lungs/pneumonia) is well constrained. The OOPs are not solely present in building water systems, have numerous modes of transmission and various infection sites as shown in Figure 1.
FIGURE 1. Waterborne opportunistic pathogens have a range of sources, transmission and infection that all need to be considered for disease prevention.
Preventing disease from these OOPs needs to involve infection prevention practices, which may include building water systems, but also considers numerous other systems and processes that a patient or building occupant interacts with. Facility-specific information from infection preventionists and resources, such as APIC’s “Ready Reference for Microbes” (Chachere, 2018), should be consulted to understand more about OOPs of concern in healthcare environments.
If the program team has determined a waterborne pathogen in addition to Legionella requires management, the design team needs to carefully coordinate the WSMP with the organism(s) of concern and the building design details. Some examples of these items are as follows.
Fixture selection: Peer-reviewed literature has evaluated the growth of pathogens in manual fixtures vs. electronic sensor fixtures, and recently manufacturers have developed improvements to sensor fixtures, handle blades and materials to minimize pathogen growth. Fixture selection also needs to consider splashing and drain interactions to avoid release of pathogens onto surrounding surfaces. Even the location of soap and towel dispensers should be considered to avoid environmental sources for pathogen growth and contamination of surrounding surfaces.
POU filters: Point of use filters provide a barrier to transmission of many waterborne pathogens. POU filters may be used on sink fixtures, showers or in-line to supply ice machines or other equipment. If POU filters will be installed or could be installed as part of the WSMP on sink fixtures, the design team needs to include provisions to allow their connection to fixtures, minimize splashing if offsets change, ensure proper hand clearance remains and ensure the placement doesn’t overlap with any sensor eyes. A maintenance schedule is also required to ensure timely replacement per manufacturer’s requirements.
Supplemental disinfection: Supplemental disinfectants considered for Legionella (such as ionization, monochloramine, chlorine dioxide or chlorine) may not have the same efficacy on other waterborne pathogens. For example, peer-reviewed literature indicates ionization is very effective for Legionella, but not as effective for pseudomonas or nontuberculous mycobacteria.
Commissioning and shock disinfectants: The planned disinfectant used for commissioning or considered for shock disinfection in the WSMP needs to be coordinated with the water system design. Wetted coatings and elastomers may not have the same compatibility to high levels of certain oxidants used in commissioning or shock disinfection. In addition, the optimal disinfectant, concentration and contact time may vary depending on the pathogen of concern.
Commissioning validation: As noted earlier, there is no surrogate test for opportunistic pathogens. If commissioning is performed to minimize pathogen growth prior to building occupancy, the specifications need to require environmental testing for the specific organism, and an appropriate sampling program designed to validate efficacy of the commissioning disinfection process.