Editor's Note: The footnotes denoted in this article are listed at the end under "References."
Recent reports of whirlpool-associated septicemia(1), skin infections(2), urinary tract infections(3), pneumonia(4), legionellosis and Pontiac fever(5,6) have raised serious public health concerns about the risks associated with whirlpool bathtubs. To assess these risks, whirlpool bath water samples were aseptically collected from private homes and hotels from across the United States. This article deals with whirlpool bathtubs that are filled and drained after each use, as distinguished from recreational spas and hot tubs.
A typical whirlpool bathtub incorporates a system of inaccessible air and water piping(7). When a bather fills the tub and activates the system, normal flora, dirt, sloughed skin, body fluids, bath oils and additives, fecal matter and soap scum circulate through the system and build up inside the piping as biofilm. Biofilm is abundant in nutrient containing aquatic environments, and due to physiological cooperation, they are inherently more resistant to various antimicrobial treatments and cleaning methods. Manufacturers recommend flushing the system with automatic dishwasher detergent, bleach, vinegar or baking soda(8,9,10), but the effectiveness of those products is highly doubtful (Hendrickson, Connie M. Ph.D., personal communication).
Most systems permit dirty bath water to back-fill the air piping when the pump is turned off. Unlike the water circulation piping, the air piping will not admit fluid while the pump is operating. Even if industry-recommended cleaning agents were effective, they cannot reach the air piping, which makes the complete system uncleanable by any means(7).
Although the tub is drained after use, it appears that the circulation system itself does not fully drain. The industry standards committee of the American National Standards Institute has adopted a standard that permits the typical circulation system to retain over 10 fluid ounces of dirty bath water when the bathtub is fully drained(11). Stagnant bath water trapped inside a system already rich in biofilm provides an ideal environment for infectious bacteria to flourish.
These factors combine to expose the bather to pathogenic organisms. The hazardous effects are compounded by the fact that these organisms are delivered in aerosol form due to aeration of the water through jets.
The Study
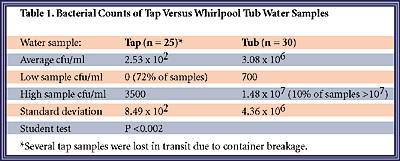
Whirlpool bath samples were collected from all over the United States from both private homes and hotels, and were subjected to bacterial analysis. Aseptic technique was used to collect both tap and tub samples into sterile 100-ml water collection containers(12). One container of tap water was collected after the tap was allowed to run for 1-2 minutes and four containers of tub water were collected after a clean tub was filled and the jets were engaged for 2-3 minutes. Bacteriological examination of the water first involved nutrient agar pour plates of water dilutions to assess relative bacterial numbers. Secondly, 100-ml volumes of water were filtered through a membrane filter with a pore size of 0.45 um, and the filters were then placed on Eosin Methylene Blue (EMB) agar, Mannitol Salts agar (MSA), Pseudomonas F agar, Buffered Cysteine Yeast Extract (BCYE) agar and Sabaroud agar. All the plates were incubated for 24-28 hours at 37|C, except for EMB, which was incubated at 44|C. By elevating the incubation temperature to 44|C for this group, many of the non-enteric coliforms are eliminated.Findings indicate that, as compared to tap water samples, the bacterial numbers were greatly increased in the whirlpool tub samples (253 cfu/ml vs. 3.08 x 10 to the 6th power cfu/ml, respectively, P <0.002)(See Table 1.) Normal (nonjetted) tub water samples were not significantly different from tap water samples. Additionally, all whirlpool tub samples yielded microbial growth, whereas 72% of the tap samples showed no growth under the experimental conditions used in this trial. No data correlating the number of viable organisms in water with the risk of acquiring infection is currently available. However, the analysis of 100-ml filtered samples yielded TNTC (too numerous to count, or >300 cfu) in 61% of the tub samples tested, indicating that the bacterial load for a 100-ml sample was fairly high in a majority of cases.
Observance of the plated filters yielded the results listed in Table 2. Growth on EMB was followed by the use of Enterotube II, a commercial testing system (Remel) for identification of gram negative rods belonging to the Family Enterobacteriaceae. Escherichia coli, Proteus mirabilis, Yersinia pseudotuberculosis, Shigella sp., Serratia sp. and Klebsiella sp. were among the organisms identified in this group. Gram positive cocci that formed yellow colonies on MSA followed by a positive rabbit plasma coagulase test confirmed the presence of S. aureus. Pseudomonas F agar and OxiFerm tubes (Remel) were used to identify the presence of various Pseudomonas species. Sabaroud Dextrose agar and colony morphology indicated the presence of fungi. Buffered charcoal yeast extract agar with PAV was used for enhanced growth of presumptive Legionella species. (Vancomycin inhibits gram positive organisms and polymyxin B inhibits many gram negative bacilli; Anisomycin suppresses yeast.) Legionella pneumophila produces green colonies and Legionella micdadei produces blue colonies.

Conclusions
Association of infections with whirlpool tubs has been recognized for a number of years, but due to the increased popularity and the use in hydrotherapy(13), the matter should be brought to public attention. A previous study has shown the colonization of whirlpool baths withP. aeruginosaregardless of "the type of whirlpool bath, its length of time in use, exclusion of residents with incontinence, infection or skin problems, type of disinfection or method and frequency of disinfectant used, and whether the bath was serviced regularly"14. The results of the Hollyoak study prompted the Public Health Laboratory Service Water Committee in the U.K. to further investigate the link between the use of whirlpool baths and infections so that health guidelines can be established. Likewise, the Dutch government has launched a plan to combat Legionnaire's Disease by implementing water safety measures after 242 cases of Legionnaire's Disease developed due to exposure to aerosolized bacteria from a whirlpool bath at the Westfriese Flower Exhibition in the Netherlands in February 1999(15).
Due to the presence of pathogenic and potentially pathogenic organisms, education of the public on the hazards of piped whirlpool bathtub use should become a priority. Immunocompromised and post-operative individuals should discontinue use, and all individuals should avoid submersion of the head and possible ingestion of the water. Another concern, particularly in the hospital setting, is that a whirlpool bath could act as a reservoir of antibiotic resistant microorganisms. New technology in design and the use of professional cleaning systems would be beneficial in reducing the risks associated with whirlpool tub microbial exposure.
Acknowledgements
The technical assistance of Stephen Bell and Kim Orr was greatly appreciated. I would also like to thank the National Council for Whirlpool Bath Health and Safety for its assistance in sample collection for the survey.References
1. Holmes RL, Kozinn WP. DF-2 septicemia following whirlpool spa immersion. J Clin Microbiol 1986;23(3):627-628.2. Berger RS, Siefert MR. Whirlpool folliculitis: a review of its cause, treatment and prevention. Cutis 1990; 45(2):97-98.
3. Salmen P, Dwyer DM, Vorse H, Kruse W. Whirlpool associated Pseudomons aeruginosa urinary tract infections. JAMA 1983;250(15):2025-2026.
4. Rose HD, Franson TR, Sheth NK, Chusid MJ, Macher AM, Zeirdt CH. Pseudomonas pneumonia associated with use of a home whirlpool spa. JAMA 1983;250:2027-2029.
5. Anonymous. From the Centers for Disease Control and Prevention. Legionnaire's Disease associated with a whirlpool spa display-Virginia, September-October 1996. JAMA 1997;277(9):705-706.
6. Fallon RG, Rowbotham TJ. Microbiological investigations into an outbreak of Pontiac fever due to Legionella micdadei associated with use of a whirlpool. J Clin Pathol 1990;43(6):479-483.
7. Guimond M. Bacterial Health Hazards in Whirlpool Baths. ASHI Technical Journal. 1993;Spring:42-44.
8. Kohler Co. A guide to care & cleaning KohlerR plumbing products. 1997 Kohler Answer Center.
9. Jacuzzi Whirlpool Bath. Builder bath series Installation/Operating Instructions.
10. Ultra Bath Instruction Manual. Usage Tips and Maintenance. 1996. 11. ASME/ANSI A112.19.7M-1995 Section 5. Whirlpool Bathtub Appliances. An American National Standard. 1995.
12. Eaton AD, Clesceri LS, Greenberg AE. Eds. Standard methods for the examination of water and wastewater. Ameri-can
Public Health Association. Washington, DC. 19th Ed. 1995.
13. Tredget EE, Shankowsky HA, Joffe AM, Inkson TI, Volpel K, Parachych W, Kibsey PC, Alton JD, Burke JF. Epi-demiology of infections with Pseudomonas aeruginosa in burn patients: the role of hydrotherapy. Clin Infect Dis 1992;15(6):941-949.
14. Hollyoak VA, Freeman R. Pseudomonas aeruginosa and whirlpool baths. Lancet 1995;346(8975):644.
15. Sheldon T. Dutch launch plan to combat Legionnaire's Disease. BMJ 1999;318:1437.

Protocol for Collection of Whirlpool Bath Samples for TAMU Research Project
Five bottles are needed from each whirlpool bath being evaluated (four for bacterial typing and one for a tap water baseline).To collect the samples, follow these steps:
- As you prepare for specimen collection, place sterile gloves on both hands. Be careful no to misplace the Sodium Thiosulfate tablet located inside the specimen containers.
- Collect a tap water sample using one of the 100-ml containers. Turn on the faucet and allow the water to run for 1-2 minutes prior to collecting. Fill the container, insert the strap through the lid and seal the container by wrapping Parafilm around the sides of the lid.
- Label the Sampling Point "Tap Water."
- Fill the whirlpool bath and activate the pump. Allow the pump to operate for 2-3 minutes.
- Collect four 100-ml samples. Hold the container by the lid and dip it into the center of the bathing area.
- Insert the strap through the lid and seal the containers by wrapping Parafilm around the sides of the lid.
- Record the bath location (e.g. room number, master bath, guest bath, 4th floor hospital) as the Sample No. on each label.
- Record the date and time of sample collection on each container; below record your initials and the name and address of the facility where the whirlpool bath is located.
- Record either EMB 44¶C, Pseudomonas, MSA or Legionella on one bottle each (total of four) on the "Other" line. Record "Whirlpool Bath" as the sampling point on each of these four samples.
- Insert the containers into protective packaging (preferably a box) and seal the package.
- Arrange pick up by an overnight shipping service for next morning delivery to the Microbiology Department at Texas A University. If the sample will not be sent for next morning delivery, it must be refrigerated. Samples collected on Friday must be refrigerated and held for pick up on Monday or the next business day.

