
Figure 1. Cross-Section Through A Typical Pump.
There have been many articles and publications written about cavitation. It is my intention in this piece to discuss in practical but complete terms its causes, effects and prevention as it pertains mostly to plumbing and allied systems. What exactly is cavitation? Cavitation results from the considerable energy of vapor bubble implosions that chip (or corrode) the metal of the valve seat or pump impeller, causing cavities. Therefore, its name, cavitation.
Cavitation can occur only in liquids where the atmospheric pressure on a liquid drops below its vapor pressure, causing a small portion of liquid to temporally vaporize into vapor bubbles. Soon after the bubbles form, as they move along their path from a lower pressure toward a higher pressure, the bubbles will rapidly collapse (or implode) back into the liquid state. As the pressure increases downstream, the resulting shock wave of the imploding bubbles could cause damage.
When a flowing liquid in a pipe or enclosure passes through a restriction, an equal amount of liquid is also flowing through all areas of the piping. The smaller sectional area of the restriction has the liquid flowing at a higher velocity and a lower pressure level as it passes through and then leaves the restriction. At the point of restriction, the velocity increases. Downstream of the restriction, there is a lower pressure caused by energy losses. This is where there is a pressure drop. This is illustrated inFigure 1, where the cross section of a pump shows where cavitation will occur.
How It Develops
Before cavitation can start, the first thing that will happen is that boiling must take place. Boiling is when there is a change in state from a liquid to vapor bubbles. If the flowing velocity of any liquid in a closed system is high enough, an inline contraction will cause the pressure within the liquid to fall below the vapor pressure. This will cause the liquid in the system to change state and boil. Boiling, and the formation of the vapor bubbles, will occur because the pressure energy of a liquid reaches a lower pressure than that of the surrounding water.Liquid water and water vapor are composed of the same molecules. The difference between them is in the energy level of the molecules and the larger volumes they occupy because of their difference in state. The vapor molecules have a much higher energy level and wider movement, and, therefore, require a much larger volume than does the liquid.
The pressure energy in a liquid is converted to kinetic (flowing) energy at a pump impeller or a valve restriction, causing an increase in velocity. If this occurs, the liquid would turn to vapor and boil at this location. Most of the time we tend to associate this boiling action with the addition of heat, but in relation to pumps and valves, a reduction of pressure is often a large enough factor for formation of these bubbles without any heat added to the system. Under certain circumstances, water can boil at any temperature by lowering the pressure sufficiently below that of the vapor pressure of the liquid.
At sea level, where water boils at 212°F, the volume of the vapor is 1,600 times that of the liquid at the same temperature. When it is free to rise to the surface, vapor bursts and releases both heat and pressure energy, with heat being the major component of this energy release. This is called flashing. The shock wave generated by the burst is very small because the bubble is only slightly more than one atmosphere and the energy release is in all directions above the surface of the water.
If there is a reduction of pressure on the water’s surface, the temperature of the boiling point is reduced proportionally. If you increase the pressure on the surface of the water to more than one atmosphere, the boiling point will increase proportionally. If the increase in pressure occurs during the boiling process, it can stop the vapor bubbles from bursting. Otherwise, it will collapse and return to its original liquid state.
If the pressure is low enough at the restriction, vapor bubbles form and then collapse a fraction of a second later, as they enter a region of slightly higher pressure. When this increase in pressure allows a “pressure recovery” to occur, the vapor bubbles collapse and return to a liquid state. When the vapor bubbles collapse, its energy release is far different from that of a burst. Unlike a vapor bubble formed by boiling that bursts on the surface, a collapsing bubble below the surface actually changes back to a liquid state. Although heat is a minor part of this change of state, the shock waves generated by the collapse are the major forces generated.
Shock waves are formed by the collisions of the surrounding water molecules that rush in to fill the voids caused by the collapsing bubble, and several factors contribute to the waves intensity. Research studies have found that from formation to collapse is about three one-thousandths of a second, which is a very rapid period of time. The more rapidly the surrounding water collides, the greater the energy. In addition, if the temperature of the liquid increases, the likelihood of cavitation increases because of the increased vapor pressure. The actual size of the cavitation bubble is 35 times larger than the boiling bubble. This larger size means that there is a larger mass of water molecules to collide.
Together, velocity and mass represent the total kinetic energy of the collapsing bubble. The high velocity due to the quick collapse and the increased mass due to the vapor bubble size results in unusually high energy. But this is not the entire picture. The shape of the bubble itself plays an important part of the picture. Experimental photographs show that the bubble is not round, but rather starts with an indention called a “re-entrant micro jet” that forms on the bottom of the bubble. This jet directs the force of the collapse in a single direction. The combination of highly concentrated energy and focused direction is what makes a collapsing bubble so destructive. Even if the bubbles collapse well above the surface of the impeller and erosion is avoided, the shock wave can still cause severe vibration, which can lead to other forms of pump damage such as pitting.
Research has shown that when the bubbles collapse near a solid boundary, like an impeller vane or shroud of a pump, the direction of the micro-jet will almost always be directed to that boundary. This means that the entire energy of collapse is directed towards a small area of the impeller’s surface. This is where the metal corrosion and cavities occur.
Cavitation in Pumps
Centrifugal pumps begin to cavitate when the suction head is not sufficient to maintain pressures above the vapor pressure through the flow passages. This includes reduction of NPSHa resulting from the installation of a filter or strainer downstream of the pump. The most sensitive areas usually are the low pressure sides of the impeller vanes near the inlet edge of the pump and also near the edge of the front shroud where the curvature is greatest. Actual damage to the impeller depends on two factors:*Liquid Thermodynamic Properties.As the amount of vapor bubbles increase under operating conditions, they do more damage when they collapse. Cold water does more damage than hot water.
*Impeller Material.Steel, cast iron and brass are more susceptible to damage than impellers of stainless steel, bronze and aluminum.
The extent of the cavitation depends mainly on the downstream pressure and the differential pressure across the pump or valve. The materials used for the impeller have a resistance to cavitation pitting rating. A representative number showing a weight loss after two hours of tests are:
The value of elastomeric coatings on impellers has been known for many years. These coatings have been shown to be highly resistant to cavitation pitting, but have not been extensively used because of technical difficulties that prevented achieving an adequate bond. New technology now permits the components to be properly coated. These coatings, which are costly, should be considered for reducing pitting as a possible solution to the problem.
Cavitation in Valves
Cavitation in valves has never been a major concern to the plumbing engineer because of the relatively low pressures in a system. This does not mean that problems could never occur.The major concern in valve-throttling applications is potential cavitation. If excessive pressure drops are created at any particular closure position, cavitation could occur. As the pressure energy is recovered and rises above the vapor pressure of water (which is approximately 0.5 psia for normal temperatures), the vapor pockets that were created through the reduced orifice implode. This is schematically illustrated inFigure 2. Cavitation is not a characteristic of any one particular valve, but is possible through any valve when the pressure drops to below the vapor pressure. Certain valves, according to their characteristics, will cavitate at different closure positions for each of the various types of valve. Cavitation could occur at the following limits that will vary slightly, depending upon local conditions (seeEquation 1):
Equation 1
2g x (H + 33)
C = ----------------------
V2 + 2g x ∆ H
Where:
C = Cavitation Constant (up to 2.5 based on tests by H. Blouler & Escher-Wyss).
[If C shows less than 1.0, serious cavitation could occur. Values from 1.0 to 1.5 indicate that cavitation is possible. Above 1.6, cavitation will not occur.]
g = gravitational constant (32.17 ft/sec/sec)
H = downstream head, ft. water
V = water velocity, ft./sec.
∆ H = head loss across valve, ft. water
If there is any reason to believe that cavitation could be a problem, a quick solution to Equation 1 would indicate that a check with the valve manufacturer would be appropriate.
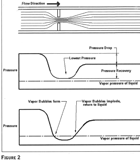
Figure 2. Schematic Illustration Of Flow Through A Valve.
Designing To Reduce Cavitation
With information available from the valve supplier, it is possible to select an optimum valve type for an unusual condition. It may be possible for pressure control applications to fit two or more high-recovery valves in tandem in the system within the building, each taking an equal fraction of the proposed drop. In addition, some valve manufacturers have developed extremely effective trim or valve internals that will combat both the onset and effect of cavitation.Bibliography
Holtgraver, E., “Cavation 101”Valvemagazine, Winter 2007.Evans, J., “Why Cavation is Damaging,”Pumps and SystemsMagazine, January 2007.
Karassik, I., et al “Pump Handbook” McGraw-Hill, New York, New York.