
The term "water purification" has a variety of meanings that depend on the industry and application involved. This article will summarize the main methods of water purification and the major classifications of water quality. To properly design a water purification system, you should start with the end in mind, which would be an industry standard, a manufacturer's recommendation or simply a client's desire for level of purity. Let's start with the health care industry, which has the most basic water purification requirements.
Hospitals
Hospitals need water that has been treated and/or purified for purposes such as sterile processing of surgical instruments, hemodialysis centers and for laboratory work. Water hardness is an important factor when considering the cleaning, disinfecting and sterilization of surgical instruments. In extreme cases, the formation of calcium carbonate crystals could trap bacterial spores, allowing them to survive the sterilization cycle. In rare circumstances, the local water supply is sufficient so that no treatment is required to meet these process needs. In other instances,water softeningis required to reduce the water hardness to the desired level, which is usually 1 to 2 ppm total hardness. Hardness is the measure of the total calcium, magnesium, iron and other metallic elements in the water. Water softening is usually accomplished by passing the raw water over a bed of granular sodium cation-exchange resin. This process removes these dissolved impurities that cause hardness by replacing them with sodium ions.
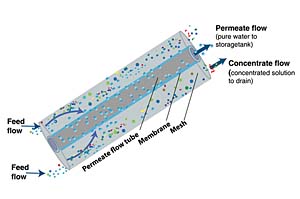
Many manufacturers of sterilization equipment prefer that the final rinsing of surgical instruments be done with reverse osmosis water. Reverse osmosis (RO) is a water purification process in which water is forced by pressure through a semi-permeable membrane. In normal osmosis, water flows from a less concentrated solution through a semi-permeable membrane to a more concentrated solution. Reverse osmosis uses pressure to reverse normal osmotic flow (see Figure 1). Water flows from a more concentrated solution through a semi-permeable membrane to a less concentrated solution. The feedwater to the reverse osmosis system flows over the surface of the membrane, and a percentage of the water is forced through by pressure and becomes the purified water or permeate. The remaining water, concentrate, retains the rejected contaminants and is drained off. The percentage of feedwater that is recovered as permeate, called percent recovery, is typically 33% or 50%. The other 66% or 50% is discharged to drain. For optimum operation of the RO equipment, the feedwater should be heated to approximately 77
Laboratories
Water quality for laboratory work varies widely depending upon the application involved. For instance, traces of organics and heavy metals are not tolerable in high-pressure liquid chromatography or in atomic absorption spectrophotometry. The laboratory will typically require what is termed high-purity water, orlaboratory-gradewater. This water will be virtually free of one class of contaminant, but may contain large amounts of other types of contaminants. The methods of reverse osmosis discussed above,deionizationanddistillationare all capable of producing laboratory-grade water. Deionized or DI water is purified by passing water through ion exchange resins that remove dissolved ionized chemicals. Deionization does not remove organic chemicals, bacteria and other microorganisms. Colonies of microorganisms can become established and proliferate on the nutrient-rich surfaces of the resin if not regularly sanitized or regenerated. Distillation will remove a wide range of contaminants through the boiling of feedwater and collecting the resulting condensate. This process is more energy intensive and is consequently more expensive to operate than the more common technique of reverse osmosis. Scale formation can also be a problem with distillation units.
The National Committee for Clinical Laboratory Standards (NCCLS), among others, has developed standards for water-used laboratories. Type I, or reagent-grade water differs from laboratory-grade water in that it is free from all classes of contaminants. It is sometimes referred to as ultrapure water, as it contains very low amounts of chemical impurities and has a very low electrical resistance. The purity of ultrapure water is about 100 or more times greater than RO water. Type II, or analytical-grade water, may be used for all but the most critical laboratory procedures. Type III, or general laboratory-grade water, is used in many quantitative analysis procedures, as well as glassware rinsing and as feedwater, for generating reagent-grade water by purifying it further with distillation or deionization. A typical application will involve a large scale RO system to generate the laboratory-grade water used throughout the facility, and then employ small, point-of-use polishing to create the reagent-grade water where it is required. The polishing usually consists of activated carbon mixed-bed deionization, followed by sub-micron membrane filtration.
Industrial Applications
Technology sectors such as pharmaceutical and semiconductor industries require large quantities of ultrapure water such that point-of-use generation is not practical. In these applications, a large central system is required that typically involves recirculation loops and storage tanks. The United States Pharmacopeia (USP) defines several types of water, of whichpurified water(PW) andwater for injection(WFI) are most common. The standards for these waters are very similar, except that WFI has a stricter bacterial count and must pass a bacterial endotoxin test. An endotoxin is a heat-resistant substance that is found in the cell walls of both viable and nonviable bacteria. Production methods for these two types of waters are also similar; however, WFI must utilize double-pass reverse osmosis or distillation. Ultrapure water is also used in the semiconductor industry to remove the residual etching acids from the surface of wafers during processing.The USP standards require that pure water be prepared with water complying with regulations of the U.S. Environmental Protection Agency (EPA) with respect to drinking water, which specify limits on coliform bacteria. This feedwater should be sampled periodically over each season of the year to measure both the microbial count and the residual disinfectant level to establish a baseline. Typical municipal water contains an adequate amount of free chlorine to limit microbial growth to satisfy EPA requirements.
Pretreatment of the feedwater usually involves a first step of filtration with a multi-media filter consisting of gravel, greensand and anthracite, which when combined can effectively remove solids as small as 5 to 10 microns. Water entering an RO system should be further filtered down to at least 5 microns to prevent clogging of the feed channel. Next, water softening is employed using ion exchange softening to protect the downstream RO system from developing scale on membrane surfaces. After softening, chlorine may be removed by either activated carbon beds or bisulfite injection. Activated carbon is more costly, and it also provides a breeding ground for bacteria. If activated carbon is used, the filter assembly is typically designed to be heat sanitized on a regular basis with either steam or hot water. Another technique employed to retard microbial growth in carbon filters is to incorporate ultraviolet (UV) light, either in a constant recirculation loop around the bed or by installing UV assemblies both up and down stream of the activated carbon.
After dechlorination, the water will often proceed to a double-pass RO, which provides an extra level of microbial reduction. As mentioned previously, the preparation of WFI requires either double-pass RO or distillation. Distillation equipment is expensive to operate due to the energy required to vaporize water. Additionally, a single-pass RO system is often employed upstream of the still to reduce the potential of scaling and fouling of the still. In the double-pass system, the product water from the first pass is used as feedwater for the second pass. Even when sanitized on a regular basis, some microbial growth will occur on the product side of the membrane. Of all the contaminants in the water supply, bacteria are the hardest to control and can live in purified water, which contains very few nutrients. The bacteria can go into a low-nutrient mode where they reduce in size and bond to the internal surfaces of pure water systems. Bacteria will attach to any surface that water contacts and develop what is termed a "biofilm."
Because of this, the pure water distribution system must be designed with features and components that work to control microbial growth. Bacteria will tend to grow in places such as threaded connections, ball valves, dead-leg piping and imperfections in pipe materials, such as extruded polyvinylchloride. So, the use of diaphragm valves, welded joints and stainless steel piping is very common. Gauges and instruments should be specified in sanitary design. The periodic use of either heat or chemical sterilization also has an impact on the selection of piping materials. Close attention should be paid to the elimination of stagnant sections of pipe as small as three to four pipediameters, as sanitizing agents cannot reach these areas, allowing bacteria to grow unchecked.
Continuous deionization (CDI), or electrodeionization (EDI), is a continuous water purification process that uses direct current, permeable membranes and a mixed-bed ion exchange resin. This technique is often used in conjunction with RO to provide water that is consistently low in bacteria. CDI equipment is sensitive to feedwater impurities and is therefore used downstream of the RO equipment for polishing purposes. It should be noted that CDI membranes and resins are incompatible with most sanitizing agents.
A storage and distribution system is employed to keep the water moving in order to discourage microbial growth. Two common options for controlling bacterial growth in the recirculation system are heat and ozonation. The use of ozone is attractive because of its relatively low capital cost compared with the equipment that would be used to heat and subsequently cool the water. Ozone also works to reduce the total organic carbon (TOC) below USP standards. The ozone can be removed by UV light, which changes the O3 to O2.