Every plumbing engineer and designer knows that hard water scale reduces valve life and performance, restricts water flow and negatively impacts water heater and boiler efficiency. The solution, ion exchange--or water softening--has been around since the 1930s, and apart from improvements in valve and vessel design, the method hasn't changed very much since its inception. All in all, it's a pretty forgiving technology.
While water softening solves a lot of problems, an increasing number of operations today require a higher degree of water quality for their processes. Reverse osmosis (RO) technology is widely regarded as the most economical and effective method for providing high-quality water for applications ranging from high-pressure boiler make-up to hospital and laboratory applications, food and beverage production and spot-free rinsing.
An RO system's performance is measured in terms of water quality and quantity, all of which are dependent on three factors, namely:
- 1. Feed water pressure
2. Feed water temperature
3. Feed water quality
Because the published performance specifications of an RO system are derived under specific temperature, pressure and water quality conditions, variance in your actual water supply must be compensated for in order to ensure proper system performance. Ignoring these factors can yield a product that falls outside of your client's requirements for water quality and quantity.
Before we examine these three factors and how to compensate for their effect, let's first identify some RO performance metrics to which we'll refer throughout this article.
Percent rejection is a measurement of product water quality, expressed as the percentage of impurities that are removed from the water, and determined by:
Percent Rejection = (Feed TDS - Permeate TDS) divided by Feed TDS
where TDS is an acronym for Total Dissolved Solids, and Permeate represents the product (high-quality) water.
Example: Your feed water has a TDS level of 650 mg/L, and your system produces a Permeate (product water) TDS of 12 mg/L.
Percent Rejection = (650 mg/L - 12 mg/L) divided by 650 mg/L = 98.1 percent
Percent recovery, a ratio of product water to feed water, is a measurement of efficiency calculated using the following method:
Percent Recovery = Permeate Flow (gpm) divided by Feed Flow (gpm)
Example: You are feeding 16 gpm into an RO, which is producing 10 gpm of permeate.
Percent Recovery = 10 gpm divided by 16 gpm = 62.5 percent
Concentration Factor (CF) is the multiple of TDS that will be found in the concentrate, or waste, stream versus that of the feed stream. It is a function of recovery, vis-a-vis:
CF = 1 divided by (1 - Percent Recovery)
From this number, we can also predict the concentrate stream TDS.
Example: Your percent recovery is 62.5%. Since the feed TDS is 650 mg/L, what will the TDS be in the concentrate stream?
CF = 1 divided by (1 - .625) = 2.7
2.7 x 650 mg/L = 1,755 mg/L
This is an important number because membrane scaling always takes place on the concentrate side of the membrane. The impact that the concentration factor will have on performance is described in detail in the "Feed Water Quality" section of this article.
Now that we've covered a few basics, we're ready to look into how the factors of pressure, temperature and water chemistry impact the performance of an RO system.
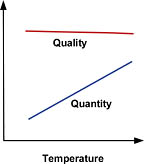
Feed Water Pressure
There is a direct relationship between feed pressure and RO performance. As feed pressure is increased, product water quality and quantity will also increase (Figure 1). Note that pressurized storage (vs. open-to-atmosphere) works against the feed pressure, so your design must compensate for this factor.Additionally, water with a high TDS concentration creates a naturally occurring osmotic pressure, which causes water to migrate contrary to the design flow of the membrane. As with the situation created by pressurized storage, this too must be compensated for in our design. As a rule of thumb, every 100 mg/L of TDS produces 1 psi of osmotic pressure. A water supply with a TDS of 1,500 mg/L, for example, would generate 15 psi of osmotic pressure (for an explanation of osmosis and osmotic pressure, see paragraph on "What is Reverse Osmosis?" ).
Taking these variables into account, to calculate the system's effectual Net Driving Pressure (NDP), we apply the following formula:
NDP = Feed Pressure (pump) - Permeate Pressure - Osmotic Pressure
Where, Permeate pressure is the pressure of your product water (in the case of open-to-atmosphere storage, we'll use 0 psi), and Osmotic pressure is based on the TDS in the concentrate (waste) stream (recall that the concentrate osmotic pressure is derived by multiplying the feed TDS by the system's concentration factor (CF) and dividing by 100).
So, if our system is driven by a pump supplying 225 psi, we are using pressurized storage set at 30 psi, and the concentrate TDS is 1,755 mg/L (determined earlier), our NDP would be:
NDP = 225 psi - 30 psi - 17.6 psi (1,755 + 100) = 177.4 psi
If the membrane is designed to operate at 225 psi, then it follows that the NDP of 177 psi (79% of design pressure) will yield a product flow 79% of that which a 225 psi NDP would produce. To put it another way, if your system has a design product flow of 15 gpm at 225 psi, expect your actual flow (corrected for NDP) to be 15 gpm x 79% = 11.8 gpm.
Assuming we are already pushing the membrane's pressure limits, increasing the feed pressure to compensate is not recommended. Instead, we should compensate by (a) adding more membranes (which will increase production capacity) or (b) utilizing open-to-atmosphere storage and re-pressurization (which, in this example, would increase the system's NDP by 30 psi). Your final choice will be determined by cost, anticipated growth, and how much "wiggle room" your original design provides.
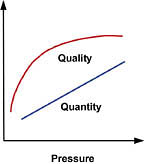
Feed Water Temperature
RO membrane performance specifications are typically derived at a feed water temperature of 77 degrees F. Temperatures less than or greater than 77 degrees F, therefore, require the application of a temperature correction factor. While temperature variations have little impact on product water quality (which decreases very slightly at higher temperatures), they can have a significant effect on the quantity produced (Figure 2). That's because as water becomes colder, it becomes denser and more viscous, requiring more energy to "push" it through the membrane. Thus, at higher temperatures, we can generate greater quantities of permeate at a given pressure and TDS than we can at lower temperatures. One solution to this problem is to raise the water temperature through the deployment of a hot-water blending valve; however, this can significantly increase operating costs, depending on the application. The other--and more common--solution is to oversize the system to compensate for the temperature's negative effect on production.Each membrane type has its own properties, so it's recommended that you consult your membrane provider with respect to temperature correction. As an example, let's assume your client requires 5,000 gallons per day of product water, and your influent (feed) stream is a chilly 45 degrees F. Applying the formula for temperature correction, where:
Desired product flow (gpd) x Tcf = Product flow at 77 degrees F
And, Desired product flow is your target production level, Tcf is the temperature correction factor (specific to each membrane type), and Product flow at 77 degrees F is the size system you will need--under the current conditions--to deliver your desired product flow.
Referencing a temperature correction table, our Tcf at 45 degrees F is 1.88. (Consult your membrane provider for temperature correction factors specific to your system.)
Applying the temperature correction factor reveals that we need a system capable of producing:
5,000 gpd x 1.88 = 9,4000 gpd
Thus, we will need a system designed to produce at least 9,400 gpd (under ideal test conditions of 77 degrees F) in order to produce our client's required 5,000 gpd under the current conditions of 45 degrees F. In other words, we must nearly double our system's capacity in order to deliver the desired product flow. Conversely, water in excess of 77 degrees F requires a smaller system than that listed in the published performance specifications to deliver your desired product flow.
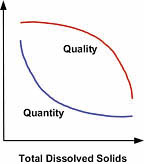
Feed Water Quality
With so many variables, the chemical make-up of water can be quite complex. However, the chief constituents to be concerned with in regard to your RO system are:Chlorine
Iron
Suspended solids
pH
Alkalinity
Calcium
Chlorine. Cellulose triacetate membranes are chlorine-resistant, but don't yield the performance of Thin Film Composite (TFC) membranes, a popular choice for most RO applications today. Chlorine will attack TFC membranes, however, so it is critical that your feed water be chlorine-free. All off-the-shelf TFC RO systems should include carbon pre-filters for the purpose of protecting the membrane.
Iron. Iron will scale a membrane in a hurry, so manufacturers recommend a limit of 0.1 mg/L in your feed stream.
Suspended Solids. Because an RO is designed to remove dissolved solids, any of the much larger suspended solids present will build up rapidly and foul the membrane (Figure 3). Water treatment professionals use a measurement known as the Silt Density Index (SDI) to determine the amount of solids in the water, and thus, the potential for plugging a membrane. Contact your local professional for an SDI assessment.
pH, Calcium and Alkalinity. The pH and alkalinity of your water supply will play a significant role, as it will influence the level at which calcium will precipitate and form scale on the membrane, limiting performance and shortening membrane life. To calculate this effect, we determine the Langlier Saturation Index (LSI) for the water. Although this article doesn't provide a detailed explanation of LSI, by definition, the LSI predicts whether the water will have scaling or corrosive tendencies. In brief, the LSI value for your system is a logarithmic function based on the concentrate stream pH, concentrate stream calcium (expressed as CaCO3) level, the concentrate stream alkalinity (the sum of bicarbonate and carbonate, both expressed as CaCO3) level, and temperature. LSI calculations require a lab water analysis.
What if your LSI indicates a potential for scaling in the membrane? This can be compensated for in one of three ways:
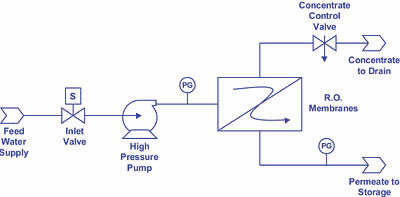
1. Reduce the recovery for the system. By sending a larger percentage of water to drain--via opening the concentrate control valve (see Figure 4)--you will in effect reduce the calcium and alkalinity levels (two of the membrane scaling culprits) in the concentrate stream. Recalling the relationship between percent recovery and concentration factor, namely:
CF = 1 divided by (1 - Percent Recovery)
it would follow that reducing the recovery would also reduce the concentration factor, which is the multiple of feed contaminants found in the concentrate stream.
2. Reduce the feed water pH through the introduction of acid feed. Your water treatment professional, membrane manufacturer or chemical supplier can aid you in this regard.
3. Pre-soften the RO feed water. A water softener will essentially remove calcium from the feed supply, therefore greatly reducing any potential for membrane scaling.
In summary, remember that when specifying an RO system, don't make recommendations based on published specifications alone. This especially holds true for a system designed for multiple locations-each with their own unique water characteristics. Properly sizing an RO system requires special attention to temperature, pressure and water quality. Unless your client's requirements leave you a lot of room to play with-or unless they have a generous maintenance budget-taking into account these "big three" factors will save you and your client many headaches in the long run.
That being said, you can make this process a lot easier on yourself by contacting your membrane supplier for a computerized sizing or RO projection program. You'll find it to be an indispensable aid.
What is Reverse Osmosis?
A natural transport mechanism known as osmosis takes place whenever two solutions are separated by a cell wall (membrane). During osmosis, the solution of lesser concentration tends to migrate through the membrane wall--which allows the solution to pass, while rejecting the Total Dissolved Solids (TDS)--into the solution of higher concentration until the two solutions are in equilibrium. The time-tested practice of gargling with salt water for sore throat relief is a practical deployment of osmotic principles. The concentrated salt solution draws fluids out of the membranes in your throat, thus reducing inflammation.In reverse osmosis, artificial pressure is applied on the concentrated solution side of the membrane, thus forcing water to migrate through the membrane wall into the less concentrated solution. The resulting solution, called permeate, is of a very high quality. Because RO employs a "cross-flow" filtration method, the remaining solids are rinsed off the membrane surface in the concentrate (waste) stream. The concentrate is usually sent to drain, but in designs where water conservation is a priority, the concentrate may be recirculated or, as in a series configuration, used to feed the next membrane. Because the membrane separates most (typically 95%-99%) of the solids from the feed stream, it follows that the waste stream contains a significantly higher TDS concentration.